Abstract
Background Venetoclax (VEN) plus azacitidine has an established role in the management of older patients (pts) with AML (DiNardo, NEJM 2020). To explore the feasibility of intensive chemotherapy combined with VEN in older pts with newly diagnosed AML, we conducted the CAVEAT trial (Chua et al, JCO 2020). We now present an updated analysis of this trial (with >3 years median follow-up), reporting on the optimal dose of VEN with chemotherapy, with and without posaconazole (POSA), the deliverability of consolidation therapy, characteristics of treatment failure and observation of substantial treatment-free remission in subsets of pts using this time-limited, short-course regimen.
Methods The CAVEAT study enrolled pts aged ≥65 years with de novo or secondary/therapy-related AML (sAML) considered suitable for intensive chemotherapy. Five VEN dose escalation cohorts (50-100-200-400-600 mg) were explored initially. Two additional cohorts examined the safety of adjusted dosing of VEN (50 or 100 mg) during anti-fungal prophylaxis with the strong CYP3A4 inhibitor POSA. For induction, VEN was administered over 14 days, commencing with a 7-day pre-chemo dose ramp-up, followed by a 7-day overlap with 5+2 chemotherapy (cytarabine 100mg/m2/d IVI d1-5 and idarubicin 12mg/m2 IV d2-3). Consolidation (CONS) with 2+1 chemotherapy (below), followed by up to 7 cycles of VEN monotherapy maintenance was planned.
Results Data cut-off: 31MAR2022. A total of 69 pts are included in this analysis (first pt 17JUL2016, last pt 15MAY2020). Median age was 71 years (range 63-80). Poor risk features included sAML (42%), adverse karyotype (28%) and prior HMA (23%).
From the dose escalation phase, the VEN 600 mg cohort was well tolerated (30-day mortality 6%). Induction with VEN-POSA (n=18) was also well tolerated (30-day mortality 0%) with 3 DLTs observed: 2 hematologic (1 in each cohort) and 1 due to pulmonary infiltrates occurring on d2 induction (suspected to be allopurinol hypersensitivity). No clinical TLS was observed. Although hematologic recovery was satisfactory after VEN-POSA induction, recovery was considerably longer in the VEN (100mg)-POSA cohort after CONS1 compared to VEN (50mg)-POSA for both neutrophils (median 64 vs 44d to 0.5 x109/L) and platelets (median not reached vs 26d to 50 x109/L). Therefore, if POSA is used concurrently, VEN 50 mg is recommended. Alternatively, if VEN 600 mg is used, POSA should commence only after completion of VEN. In the VEN 600 mg cohort, significant delays in hematological recovery were also observed after multiple rounds of attenuated CONS (VEN d1-14, bolus doses of cytarabine 100 mg/m2 d1-2, idarubicin 12 mg/m2 on d1), limiting deliverability (median CONS cycles 1; range 1-3).
By intention-to-treat, the overall response (CR/CRi) rate was 73%. CR/CRi was 90% in de novo AML (vs 51% in sAML), 84% in intermediate karyotype (vs 69% in adverse), 76% and 83% in NPM1 and IDH2 mutant AML, respectively. With a median follow-up of 37 months (m), the median overall survival (OS) was 15.4m. Median OS was 31.3m in de novo AML (40% alive at 36m) and 30.3m in intermediate karyotype AML (38% alive at 36m). In the VEN 600 mg cohort, median OS was 24.1m (36% alive at 36m).
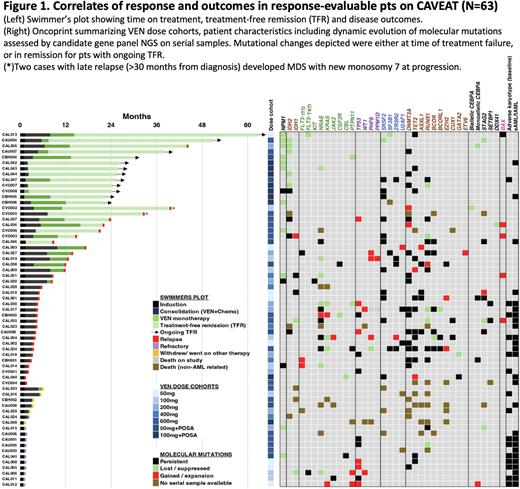
As CAVEAT delivered just one intensive cycle of therapy, followed by limited post remission therapy (median time on therapy 7.7m), we next assessed the duration of treatment-free remission (TFR) among responders. Among pts achieving CR/CRi, 20/51 (39%) experienced TFR (Fig 1). The median TFR was 22.1m (range 4.6-48.3m). At data cut-off, 12 (24%) have ongoing TFR. Notably, 15/20 (75%) with TFR had an NPM1 or IDH2 mutation at diagnosis. 11 out of 14 evaluable pts with NPM1 mutation had mutation clearance by RT-qPCR, including 3 with NPM1 negative disease at time of failure (1 refractory, 2 relapse). Two pts with persistently mutated NPM1 at relapse had a concomitant FLT3-ITD or KIT mutation. The mutation landscape at relapse is shown (Fig 1)
Conclusion VEN combined with modified intensive chemotherapy achieved high response rates in older pts with newly diagnosed AML. With long-term follow-up, the time-limited CAVEAT schedule enabled pts, especially with mutated NPM1 or IDH2, to experience a durable treatment-free remission. Cumulative hematological toxicity is limiting, prompting us to now explore non-anthracycline CONS (low dose cytarabine 20mg/m2 SC d1-10 + VEN 400mg d1-7, for up to 4 cycles), followed by VEN monotherapy (400mg D1-14 q28d cycle x7).
Disclosures
Reynolds:Abbvie: Research Funding; Alcon: Current equity holder in publicly-traded company; Novartis: Current equity holder in publicly-traded company. Tiong:Servier: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Amgen: Speakers Bureau. Fong:Abbvie: Consultancy, Honoraria, Speakers Bureau; Otsuka: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria; Astellas: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Fleming:BMS: Consultancy, Honoraria, Speakers Bureau; Gilead: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau; AbbVie: Honoraria, Speakers Bureau. Roberts:The Walter and Eliza Hall Institute: Other: AWR is an employee of WEHI which received milestone and royalty payments related to venetoclax, and is eligible for financial benefits associated with these payments.; AbbVie: Patents & Royalties: I am an inventor on a patent assigned to AbbVie related to venetoclax. Please note that I am not remunerated for this, nor eligible for financial benefit.. Wei:Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene-BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; Agios: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: Employee of the Walter and Eliza Hall Institute and is eligible for a fraction of the royalty stream related to Venetoclax, Research Funding; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees.
OffLabel Disclosure:
Venetoclax is a BCL-2 inhibitor that is FDA-approved in some indications. This presentation will focus on correlative study results in the CAVEAT trial which combines venetoclax with modified intensive chemotherapy in AML, which is not an approved indication.
Author notes
Asterisk with author names denotes non-ASH members.